On October 10, 2019, the FDA approved the use of 18F-DOPA in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for the evaluation of adult patients with suspected Parkinsonian syndromes (PS).
This approval came more than a decade after 18F-DOPA usage has been approved in Europe.
Fluorodopa (18F-DOPA) is a radioactive diagnostic agent indicated for use in positron emission tomography (PET) in Neurology to assess the integrity of the brain dopaminergic system, in the striatum, for the evaluation of adult patients that exhibit Parkinsonian features.
6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine (18F-DOPA ) is a radioactive diagnostic neutral amino acid that is transported into presynaptic neurons, where it is converted by the enzyme aromatic amino acid decarboxylase (AAAD) into 18F-fluorodopamine, which subsequently enters the presynaptic catecholamine-storage vesicles.
18F-DOPA crosses the blood-brain barrier, therefore, when injected into the blood stream, it reaches the dopaminergic cells in the brain and is used by the brain as a precursor for dopamine.
This makes it possible to monitor intracerebral synthesis and uptake of dopamine by means of the positron-emitting 18F-DOPA with PET-CT camera.
The main reason for using 18F-DOPA PET imaging is that parkinsonian subjects who have normal dopaminergic imaging do not have evidence of Parkinson’s Disease (PD) or an atypical parkinsonian syndrome.
Considering that about 20% of patients diagnosed with PD are discovered false diagnosed after post mortem examination, this aspect constitutes a key challenge in helping clinicians to ascertain positive diagnosis of PD.
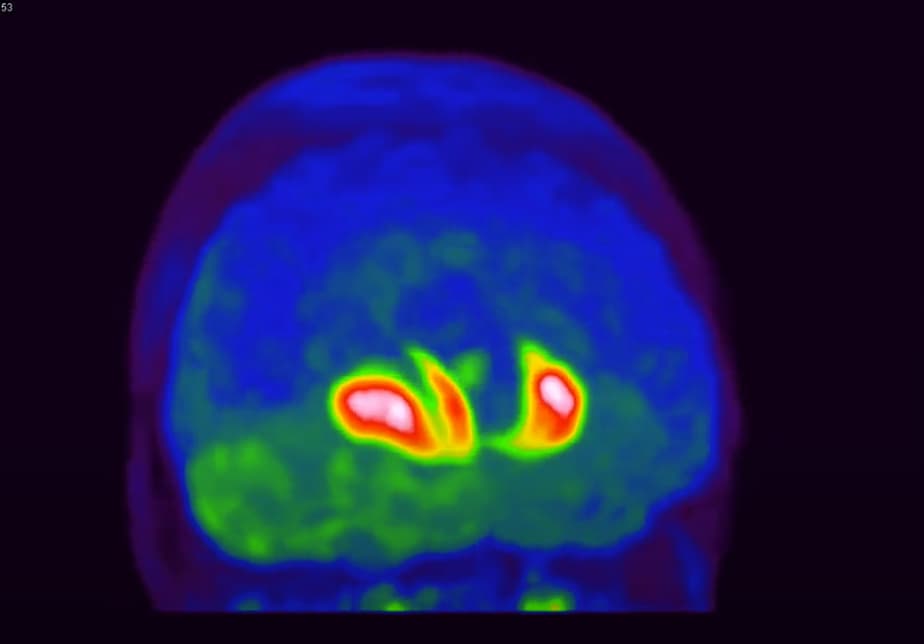
The typical pattern of 18F-DOPA uptake in PD is a severe impairment of uptake in the posterior part of the putamen, less impairment in the anterior putamen and a relatively spared 18F-DOPA uptake in the caudate (Brooks et al, 1990; Kuwabara et al, 1995; Lee et al. 2000).
Thus, the use of 18F-DOPA is a must for correct diagnosis and subsequent management of potential PD patients.
How early can 18F-DOPA be used to demonstrate dopaminergic deficit?
Probably a decade or more prior to motor symptoms;
iRBD (idiopathic REM sleep behaviour disorder) is one of the earliest non-motor signs for suspected PD.
iRBD is a parasomnia where the physiological atonia during REM sleep is absent or greatly diminished and is characterized by dream-enacting behaviour associated with nightmares.
Observational studies, have suggested that most patients with iRBD will eventually develop a defined neurodegenerative disease, almost always diagnosed as synucleinopathy.
The latency from iRBD to defined Parkinson’s disease, dementia with Lewy bodies, or MSA averages over 10 years.
A paper by Stokholm MG et al., published in Lancet Neurology, where 19 patients with IRBD and no clinical evidence of parkinsonism and cognitive impairment were recruited from tertiary sleep centres in Spain (Barcelona) and Denmark and were assessed for neuroinflammation with 11C-PK11195 PET tracer, and for dopaminergic function in the putamen and caudate with 18F-DOPA PET.
18F-DOPA uptake was reduced in IRBD in the left putamen and right putamen, but not in the caudate.
Thus, subjects with iRBD already shows striatal dopaminergic neurons deficit, this provides an unprecedented opportunity to directly observe prodromal neurodegenerative states, and potentially intervene with neuroprotective therapy.
Since 2012, ISOTOPIA MOLECULAR IMAGING is providing the neurology and diagnostic community in Israel with 18F-DOPA for PET-CT imaging and contributing an important measure for reducing the misdiagnosis of PD.

Dr. Haim Golan
Medical Advisor