
AN ADVANCED PSMA-11 RADIOLABELING KIT FOR PROSTATE CANCER IMAGING
SIMPLICITY MEETS DIAGNOSTICS EXCELLENCE
Isoprotrace® is available in the Netherlands, Germany, the UK, Sweden, Denmark, Greece and Malta
Product Overview
Isoprotrace® is a ready-to-use, multi-dose kit for the efficient preparation of Gallium (68Ga) Gozetotide solution for I.V. injection.
Isoprotrace® providing a valuable tool for enhancing diagnostic accuracy and patient care. It is indicated for use in Positron Emission Tomography (PET) to detect PSMA-positive lesions in men with prostate cancer, providing a valuable tool for enhancing diagnostic accuracy and patient care.
Active ingredient: 10µg Gozetotide (PSMA-11)
Presentation: Multi-dose sterile vacuum vial
The Package contains: 5 vials
Shelf life: 2 years
Key Features: Fast, Efficient, Reliable
Easy Multi-Dose Preparation:
Economically attractive, multi-dose preparation performed in just 5 minutes
Time and Resource Efficient:
Minimal product loss due to faster preparation time and minimal handling.
Single Sterile Vacuum Vial:
Ready-to-use, simplifies the labeling process.
High-Precision PSMA Biomarker:
Contains PSMA-11 for precise prostate cancer diagnosis.
Simplified QC:
RCP with iTLC; no HPLC needed, fewer tests required.
Quality Assured:
Manufactured in FDA-inspected facilities, compliant with EU GMP.
Indications
Isoprotrace® is indicated for PET imaging of PSMA-positive lesions in adult men with prostate cancer:
- Primary staging of patients with high-risk prostate cancer prior to primary curative therapy.
- Suspected prostate cancer recurrence based on elevated serum prostate-specific antigen (PSA) level, after primary curative therapy.

Understanding PSMA PET/CT
What is PSMA?
Prostate-Specific Membrane Antigen (PSMA) is a protein highly expressed in prostate cancer cells, makes it a valuable biomarker for both detecting and targeting prostate cancer.
PSMA PET/CT combines the functional imaging capabilities of PET with the anatomical detail of CT, allowing for precise localization of cancerous lesions.
Importance of PSMA Imaging
- High sensitivity and specificity for detecting prostate cancer lesions.
- Useful in both primary staging and recurrence detection.
- Aids in treatment planning and monitoring.
Advanced Imaging Capabilities
Isoprotrace® leverages cutting-edge PSMA-targeted imaging technology to provide:
- High Sensitivity and Specificity: Detect even small metastases with precision¹⁻².
- Whole-Body Scanning: Comprehensive assessment of primary tumors and metastatic spread³⁻⁴.
- Clear Visualization: High-contrast images for accurate interpretation⁵.
- Early Detection: Identify recurrence at lower PSA levels compared to conventional imaging⁶⁻⁷.
Technical Information
Isoprotrace® offers unparalleled convenience with on-site preparation

Radiolabeling
With Gallium-68 (68Ga)

5-Minute Preparation
Rapid, efficient process from
start to finish

Generator Compatibility
Fully compatible with both(68Ge)/(68Ga)
generators approved in Europe
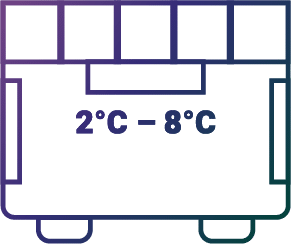
Storage
Store in refrigerator (2°C – 8°C),
before reconstitution

Quality Control
RCP with iTLC (no HPLC needed),
fewer QC tests required

Shelf Life
2 years from the date of manufacture when stored properly
FAQ
Before reconstitution: Store in a refrigerator at 2-8ºC (36-46ºF).
After radiolabeling: Stable up to 4 hours at room temperatur
The pH of the prepared Isoprotrace® solution typically ranges from 4 to 5.8.
It eliminates the need for an external vacuum vial for the Gallium generator.
Only iTLC (Instant Thin-Layer Chromatography) is required for quality control; HPLC is not necessary.
Regulatory Information
Isoprotrace® has received marketing authorization in the Netherlands, Germany, the UK, Sweden, and Denmark with pending approvals in more EU countries.
For detailed information, please refer to the product’s SmPC.
Want To Learn More?
Download the brochure
References
1. Ida Sonni, Adam B. Weiner, Sahith Doddipalli, Madhvi Deol, David Ban, Hye Ok Kim, Tristan Grogan, Preeti Ahuja, Nashla Barroso, Yang Zong, Priti Soin, Anthony Sisk, Johannes Czernin, William Hsu, Jeremie Calais, Robert E. Reiter, Steven S. Raman. Clinical, Pathologic, and Imaging Variables Associated with Prostate Cancer Detection by PSMA PET/CT and Multiparametric MRI.Journal of Nuclear Medicine Oct 2024, jnumed.124.268443; DOI: 10.2967/jnumed.124.268443.
2. HopeTA, GoodmanJZ, AllenIE, CalaisJ, FendlerWP, CarrollPR.Meta-analysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60(6):786-793. doi:10.2967/ jnumed.118.219501.
3. Al Saffar H, Chen DC, Delgado C, Ingvar J, Hofman MS, Lawrentschuk N, Perera M, Murphy DG, Eapen R. The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer. Cancers. 2024; 16(5):939..
4. HopeTA, Eiber M, Armstrong WR, etal. Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvicnodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol. 2021;7(11):1635-1642. doi:10.1001/jamaoncol.2021.3771.
5. Zhou J, Wu R, Wang W, Zhao Y, Liu X. (68)Ga-PSMA PET/CT for the evaluation of metastasis in patients with prostate cancer: a systematic review and meta-analysis. Hell J Nucl Med. (2022) 25(3):297–311. doi: 10.1967/s002449912525.
6. Kunst N, Long JB, Westvold S, et al. Long-Term Outcomes of Prostate-Specific Membrane Antigen–PET Imaging of Recurrent Prostate Cancer. JAMA Netw Open. 2024;7(10):e2440591. doi:10.1001/jamanetworkopen.2024.40591.
