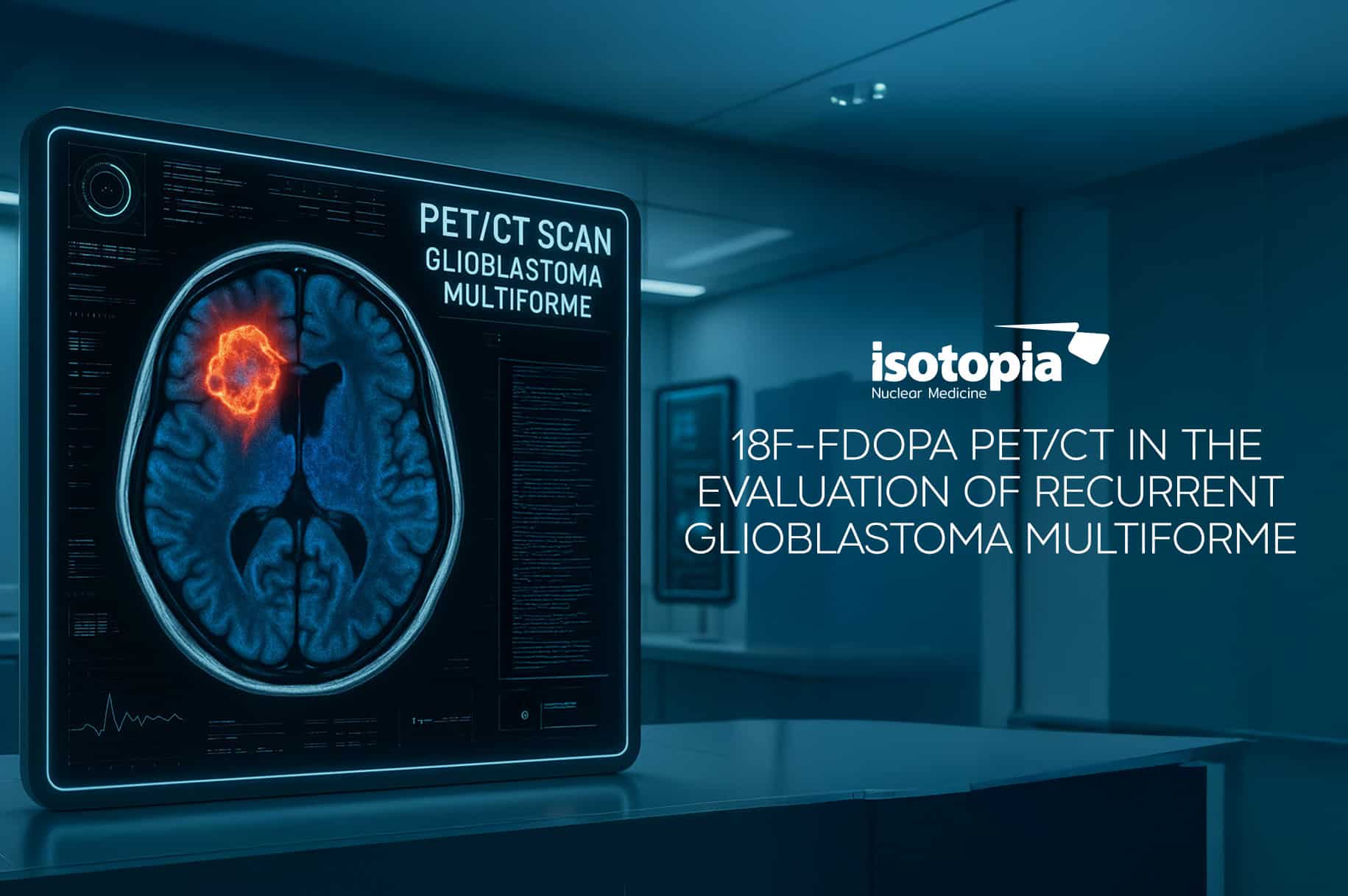
Isotopia has received the Adoption of COMMISSION IMPLEMENTING DECISION granting marketing authorization (MA) under Regulation (EC) No 726/2004 of the European Parliament and the Council for its Lutetium (177 Lu n.c.a) chloride, a medicinal product for human use.
The centralized application was approved on 15\09\2022 allowing Isotopia to access the EU with this important radioisotope for cancer care.
This two-year effort has been a joint effort with Billev Pharma as our marketing authorization holder and ABX-CRO, to support compiling the dossier, which we thank for their support and expertise.
As market access are ongoing, we are happy to share the availability already in Germany, Austria, Netherlands, and Belgium.
Isotopia is working to expand access as soon as possible to other countries to allow consistent supply to the market.