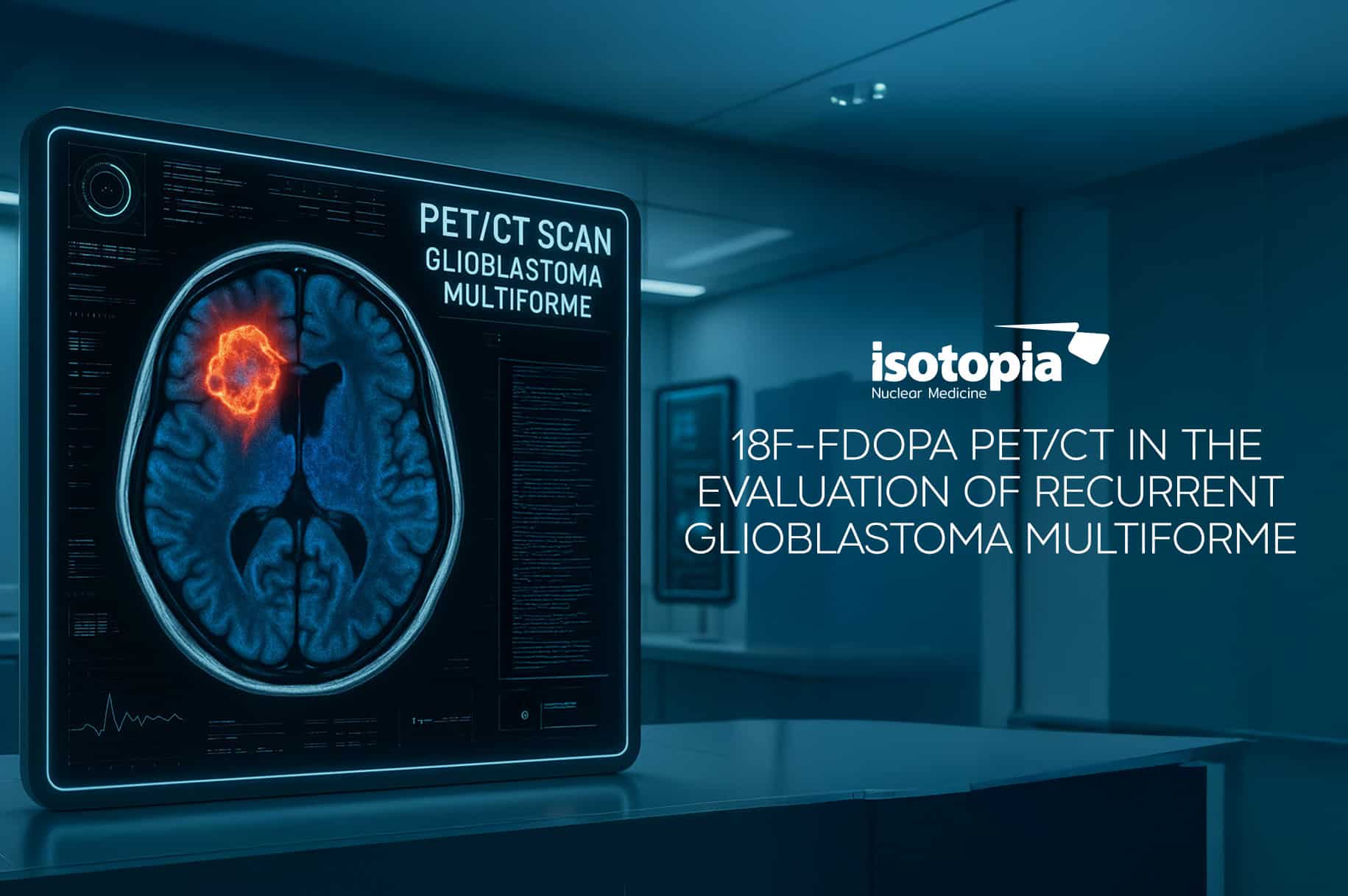
Lutathera and Lu-PSMA 617 are FDA-approved therapeutic radiotracers used to treat neuroendocrine tumors and prostate cancer.
As the demand for radioisotopes increases, producers are challenged to supply the market with sufficient products to accommodate the heightened demand without compromising product quality and patient health. In addition, several clinical studies in different clinical development stages are ongoing adding to commercial demand.
The importance of Lutetium-177 in cancer treatment cannot be overstated. Over 40 million nuclear medicine procedures are performed every year, and the demand for radioisotopes is increasing rapidly.
Lutetium-177 is a medium-energy ß-emitter (490 keV) with a long physical half-life of 6.7 days, which is helpful for synthesis and transport, and an average beta–particle range in the soft tissue of 670 nm and maximal tissue penetration of 1-2 mm. Another benefit of Lu-177 is that it produces low-energy gammas (113 keV, 208 keV), suitable for imaging purposes, allowing biodistribution and excretion kinetics to be monitored and calculated dosimetry with SPECT-CT. Lutetium-177 has favorable chemistry for chelation. Therefore, it is gaining popularity for therapy applications.
Despite its many benefits, manufacturing radioisotopes such as Lutetium-177 requires a deep level of expertise and logistical processes. From technical capabilities and infrastructure to reliable manufacturing and distribution, each phase needs to be carefully orchestrated to ensure optimal quality and “on time” supply to patients.
Choosing the right isotope supplier with a scalable solution that follows GMP practices is essential. The right supplier should also have deep regulatory expertise and the infrastructure to meet tight deadlines.
Lu-177 Transportation Challenges
One of the main challenges remaining is the transportation of Lutetium-177 over long distances. For even the most experienced pharmaceutical companies, this remains a massive hurdle. The successful transport of radioactive materials is vested primarily in logistics capabilities. That being said, especially during Covid, being closer to the customers helps de-risk logistics to ensure safe delivery for each patient. To solve this challenge in North America, Isotopia set up a manufacturing site in partnership with The Centre for Probe Development and Commercialization (CPDC) in Hamilton, Canada, which began commercial supply of n.c.a. Lutetium-177 in March 2022.
Production in Canada is designed to serve customers in North America and, address the growing need for Lu-177, and of course, save costs, transportation, and shorten patient arrival time.
Learning From The Past
Although most therapeutic radionuclides are produced in nuclear reactors, only a few nuclear reactors produce therapeutic radionuclides. Commercial production of radionuclides occurs in research reactors, which are also used for several other research or industrial applications.
Between 2008 and 2010, several reactors were temporarily shut down during what was known as the Moly crisis, during which technical glitches at a Dutch nuclear reactor that produces medical isotopes led to rationing of medical tests and treatments globally.
More recently, a shutdown at a nuclear reactor in Petten, in the Netherlands, impacted global supplies of molybdenum-99 (Mo-99) and lutetium-177 (Lu-177) for medical use. These unexpected dips in supply led to reduced shipments of medical isotopes to many hospitals.
Considering these issues from the past, seeking a supplier with a proven supply and a network backed by long-term agreements is critical. The ability to maintain weekly Lutetium-177 production ensures that future demand is covered. A stable supplier such as Isotopia is wisely engaged with a network of reactors around the globe to back up each other and guarantee the consistency of supply.
Establishing a Global Footprint
On September 8th, 2020, The Center for Probe Development and Commercialization (CPDC), a global leader in the development, production, and commercialization of radiopharmaceuticals, announced a collaboration with Isotopia Molecular Imaging for the production of n.c.a. Lutetium-177 and distribution in North America.
Isotopia’s CEO & Co-Founder Dr. Eli Shalom stated “The CPDC manufacturing site is the first step towards Isotopia’s global footprint plan to ensure efficient production of n.c.a. Lutetium-177 will be added to its existing Drug Master File (DMF). CPDC’s strategic location in Southern Ontario, its professional and enthusiastic personnel, renowned expertise in logistics, and just-in-time delivery will enhance the distribution of Isotopia’s n.c.a. Lutetium-177 and secure supply for our radiopharmaceutical and CMO customers in North America.”
Communication is Key
Communication is vital for time-critical materials such as isotopes. The “just in time” nature of the supply chain can be achieved through various communication channels. Suppliers should be a point of contact for everything from billing administration and logistics to training.
Lutetium-177 suppliers should maintain a policy of open communication. When changes are required, especially in our dynamic climate, having open communication allows for a more collaborative and streamlined process, affecting all touchpoints.
To summarize
The future of nuclear medicine is promising, and the research being done now stands to extend further our ability to diagnose and treat disease. With continued investment in nuclear medicine research, radiotracers will continue to become more specific and effective at treating disease.
Overcoming supply and production challenges to the use of Lutetium-177 is the most important action to ensure the successful implementation of this promising medical innovation.
Published by Isotopia (April 2022)