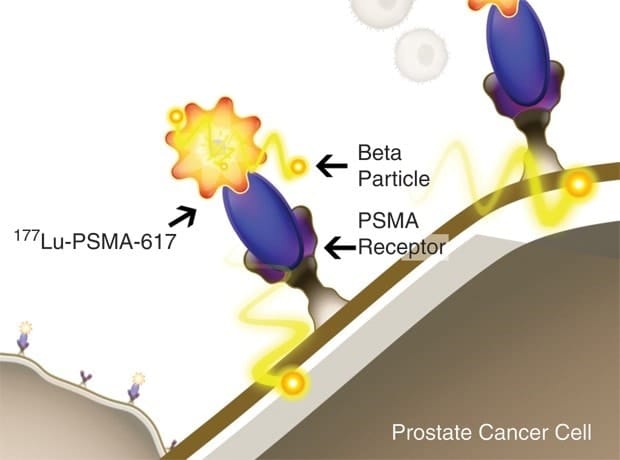
177Lu-PSMA-617 radioligand therapy in mCRPC – VISION phase 3 trial
The results from the “ProPSMA” Study, that was recently published in JAMA Oncology by Prof. Michael Hofman et al, showed that PSMA-targeted imaging with 68Ga-PSMA-11 is more accurate and valuable in guiding the management of prostatic cancer patients in comparison with the current practice of conventional imaging with CT and Bone Scan.
The theranostics pair of 68Ga-PSMA-11 is 177Lu-PSMA-617 for which a large international phase 3 study was designed, named the “VISION” trial in order to assess its efficacy over the best standard of care (SOC).
This study is sponsored by “ENDOCYTE” a US-based biopharmaceutical company focused on developing radioligand and CAR-T therapies for cancer treatment, which is owned by NOVARTIS since December 2018.
Study design:
750 mCRPC Patients with 68Ga-PSMA-11 positive scans will be randomized in a 2:1 ratio to receive:
- 177Lu-PSMA-617 plus best supportive/best standard of care
or to receive
- best supportive/best standard of care only.
VISION study graphic presentation (adopted from J Nucl Med 2019; 60:1504–1506)
The patients will be monitored for survival, disease progression, adverse events, blood tests for hematology, chemistry, PSA levels and change in quality of life.
If the VISION trial will prove the superiority of RLT arm with 68Ga-PSMA-617 over the conventional SOC arm, it will establish a new line of therapy for prostate cancer.
patients that with overtime might be applied as one of the first lines of therapy and will also become a mile stone for theranostics in precision medicine; the ability to use 68Ga-PSMA-11 based PET-CT imaging to identify patients for treatment with 177Lu-PSMA-617.

Haim Golan MD MSc
Chief Medical Officer
Isotopia LTD
ISRAEL